

Apart from that, Bohr used the Plank’s constant to calculate the energy of the energy levels of the atom.Īlthough the quantum model is much harder to understand than Bohr model, it accurately explains the observations regarding the large or complex atoms. Electrons can move from one energy level to another by absorbing or releasing energy.īohr model perfectly fit the hydrogen atom which has a single electron and a small positively charged nucleus.When an electron is moving in a certain orbital, the energy of that electron is constant.The atom is completely stable when electrons are in the lowest energy level. The smallest orbit has the lowest energy.The energy of an orbital is related to its size.where n is the fixed energy level number. Each orbit has a different radius and is named from nucleus to the outside as n=1, 2, 3, etc.The electrons move around the nucleus in spherical orbitals which have a fixed size and energy. That was suggested by observations of the line spectra for the hydrogen atom.ĭue to the presence of discrete lines in the line spectra, Bohr stated that the orbitals of an atom have fixed energies and electrons can jump from one energy level to the other emitting or absorbing energy, resulting in a line in the line spectra. This also states that these shells have different energies and are spherical in shape. But Bohr model is more advanced than the Rutherford model because it says that, the electrons are always traveling in specific shells or orbits around the nucleus. Side by Side Comparison – Bohr vs Quantum Model in Tabular FormĪs mentioned above, the Bohr model is a modification of the Rutherford model since the Bohr model explains the structure of the atom as composed of a nucleus surrounded by electrons.

The key difference between Bohr and quantum model is that Bohr model states that electrons behave as particles whereas quantum model explains that the electron has both particle and wave behavior. Quantum model is the modern model of an atom.
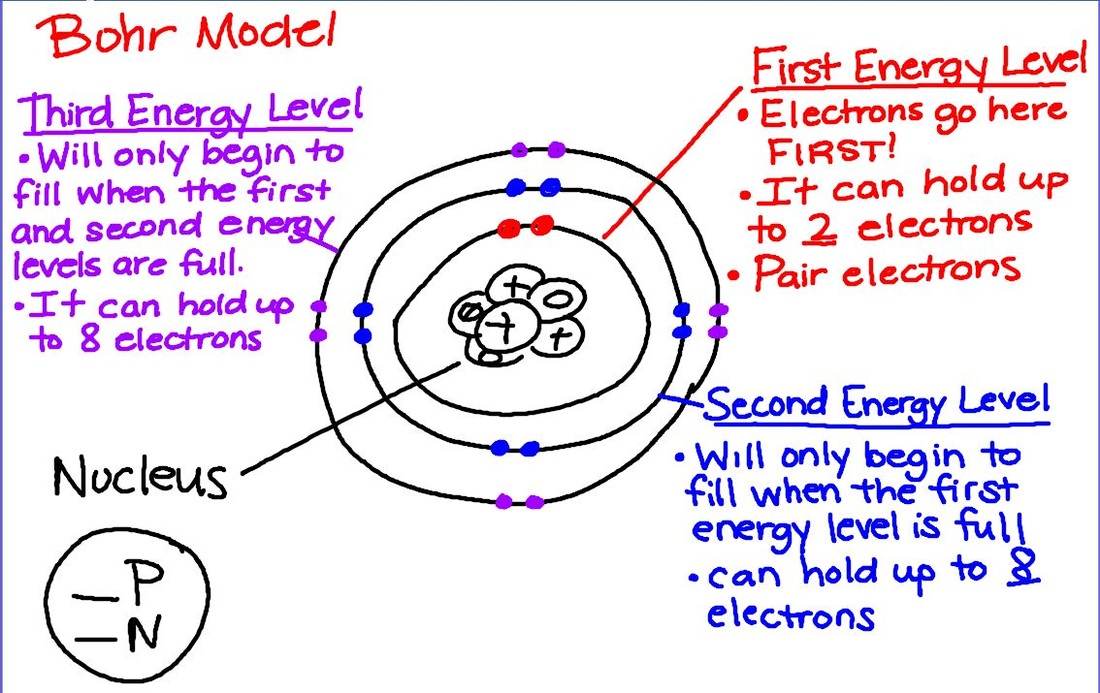
Bohr model was proposed by Niels Bohr in 1915. Bohr model is also called Rutherford-Bohr model because it is a modification of the Rutherford model. The Bohr model and quantum model are models that explain the structure of an atom.


 0 kommentar(er)
0 kommentar(er)
